Physics of the Pericellular Coat
 The focus of this research is to establish methods to measure and to study transformations of the pericellular coat during physiological events like tissue aging or cell migration in order to understand the structure-function relationship between the coat's material properties and its biology.
The focus of this research is to establish methods to measure and to study transformations of the pericellular coat during physiological events like tissue aging or cell migration in order to understand the structure-function relationship between the coat's material properties and its biology.
Attached to the surface of many mammalian cells is a layer of polysaccharides and proteins. This layer is called the pericellular coat, pericellular matrix or in some specific contexts, the glycocalyx. The thickness and constitution of the pericellular coat can vary considerably, although hyaluronan (HA) is a key structural component of most extended cell coats. HA-rich coats range in thickness from a few hundred nanometers to tens of microns thick. They appear on a variety of cells including fibroblasts, vascular smooth muscle cells, mesothelial cells, and embryonic stem cells. Not surprisingly, these thicker matrices can have a dramatic influence on cell function and its relation with the surrounding microenvironment. An often repeated but unproven statement in biological literature is that the physical properties of the cell coat, in particular its viscoelasticity, mediate key cell functions like migration and proliferation.
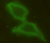
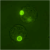
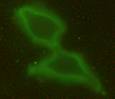
Concrete knowledge about the ultrastructural organization and the mechanics of the cell coat are lacking. For example, which of the variety of possible polymer configurations - polymer brush, multi-layered structure, crosslinked network, etc. - give rise to thicker cell coats is not understood. The most common tool to identify the coat's presence is the particle exclusion assay (PEA), a crude experiment to outline the extent of the coat by illustrating where 'particles' cannot penetrate - where typically fixed red blood cells are used due to their anti-adhesive surfaces. More recently, fluorescent probes that can label hyaluronan without collapsing the cell coat have been made available, like the GFPn construct used in our lab (gift of collaborator Uwe Rauch). Before that, the extremely hydrated cell coat (estimated 99.95% water), was difficult to probe or visualize without collapsing the structure. Transparent to bright field microscopy and altered by most staining techniques, the cell coat has remained an elusive, often ignored cell 'organelle'.
The molecular components of coats from certain cell types have been characterized. The structural backbone of the coats appears to be HA, which is anchored directly to the cell's plasma membrane via HA-binding proteins, often CD44. The molecular interaction of HA with additional HA-binding proteins varies with the tissue, and appears to determine the architecture and extent of the PCC. The resultant mesoscopic arrangement of the different PCC components influences the cell's perception and interaction of the extracellular environment and its ability to withstand compression.
In one exceptional case, the mechanical properties of the cell coat have been shown to matter - in chondrocytes, cells located in the load-bearing cartilage. The molecular structure of some cell coat components, especially the HA-binding protein aggrecan - prevalent in chondrocytes, changes with age or osteoarthritis. These changes alter the viscoelasticity of the cell coat and possibly affect its molecular architecture. Currently, few if any methods exist that characterize these mesoscopic changes in the pericellular coat induced by age, sickness or therapeutic treatments. The second objective of this proposal is to establish methods to measure and ultimately study transformations of the coat during physiological events like aging or migration to understand the structure-function relationship between the coat's material properties and the biology.